Answer:
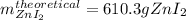
Step-by-step explanation:
Hello,
In this case, the required chemical reaction is:
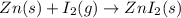
In such a way, if 125 g react with sufficient iodine in stoichiometric proportions (no excess), the theoretical yield of zinc iodide turns out as shown below:
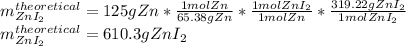
Considering that the molar ratio zinc to zinc iodide is 1 to 1.
Best regards.