Answer : The equilibrium constant expression will be,
![k_c=([Ag^(+)]^2)/([Cu^(2+)])](https://img.qammunity.org/2019/formulas/chemistry/college/y4d5bhm765gqvnxoyfv0iwwebkahj8mx64.png)
Explanation :
The equilibrium expression for the reaction is determined by multiplying the concentrations of products and divided by the concentrations of the reactants and each concentration is raised to the power that is equal to the coefficient in the balanced reaction.
As we know that the concentrations of pure solids are constant that is they do not change. Thus, they are not included in the equilibrium expression.
The given balanced chemical reaction is,
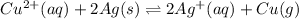
So, the equilibrium constant expression will be,
![k_c=([Ag^(+)]^2)/([Cu^(2+)])](https://img.qammunity.org/2019/formulas/chemistry/college/y4d5bhm765gqvnxoyfv0iwwebkahj8mx64.png)