Noble or inert gases belong to the group 8A and have completely filled stable, outermost shells. The outermost electronic configuration of the noble gases can be represented as,
1. Helium -
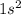
2. Neon -
![[He]2s^(2)2p^(6)](https://img.qammunity.org/2019/formulas/chemistry/high-school/7kjdct63lzhyu86nbah491hgfxmq37jfu6.png)
3. Argon -
![[Ne]3s^(2)3p^(6)](https://img.qammunity.org/2019/formulas/chemistry/high-school/bufhyxl31h8cg6o45c0w9c2r5zne7qawwm.png)
4. Krypton -
![[Ar] 3d^(10)4s^(2)4p^(6)](https://img.qammunity.org/2019/formulas/chemistry/high-school/t2i1gxdfre5vqbjsqn5rcf4jef6bhbfpkt.png)
5. Xenon -
![[Kr] 5d^(10)6s^(2)6p^(6)](https://img.qammunity.org/2019/formulas/chemistry/high-school/qlev63kpqnl6o7ye3htoqxf8zl6shh78b6.png)
6. Radon -
![[Xe] 4f^(14) 5d^(10)6s^(2)6p^(6)](https://img.qammunity.org/2019/formulas/chemistry/high-school/h9v0eydp7l7tc9wgbw5wa2h3tb1qhe81du.png)
So, the correct answer will be noble gases.