2 C₁₇H₁₉NO₃ + H₂SO₄ → Product
Moles of H₂SO₄ = M x V(liters) = 0.0116 x 8.91/1000 = 1.033 x 10⁻⁴ mole
moles of morphine = 2 x moles of H₂SO₄ = 2.066 x 10⁻⁴
Mass of morphine = moles x molar mass of morphine = 2.066 x 10⁻⁴ x 285.34
= 0.059 g
percent morphine =
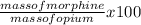
=
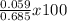
= 8.6 %