Answer: Option (d) is the correct answer.
Step-by-step explanation:
Sodium is an alkali metal and it has atomic number 11. Electronic distribution of sodium is 2, 8, 1.
Whereas chlorine is a non-metal and it has atomic number 17. Electronic distribution of sodium is 2, 8, 7.
Therefore, both sodium and chlorine needs to become stable in nature. Hence, sodium will easily lose its one valence electron and changes into
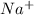 ion. On the other hand, chlorine will readily gain one electron and changes into
ion. On the other hand, chlorine will readily gain one electron and changes into
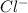 ion.
ion.
Since, charges on both sodium and chlorine are same. Hence, when both of them chemically combine together then they will result in the formation of sodium chloride (NaCl).
Thus, we can conclude that NaCl is a possible formula of a compound formed when sodium and chlorine bond.