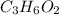Answer:
The Empirical formula of an organic compound is=
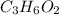
Step-by-step explanation:
Mass of an organic compound = 200 g
Mass of carbon = 97.30 g
moles of carbon =
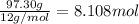
Mass of hydrogen = 16.22 g
Moles of hydrogen =
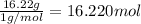
Mass of oxygen = 200 g - 97.30 g - 16.22 g = 86.48 g
Moles of oxygen =
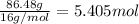
Divide the all the moles of element with smallest value of moles.
Carbon =
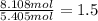
Hydrogen =
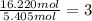
Oxygen =
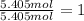
Empirical formula =
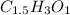
Converting them into whole number ratios.
Empirical formula =