Answer : The information we can determine from the coefficients in this balanced chemical equation is, moles.
Explanation :
According to the law of conservation of mass, the number of individual atoms of an elements present on the reactant side must be equal to the the number of individual atoms of an elements present on the product side.
The given balanced reaction is,
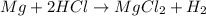
From the balanced chemical reaction, we conclude that 1 mole of magnesium react with 2 moles of hydrochloric acid to give 1 mole of magnesium chloride and 1 mole of hydrogen.
Hence, the information we can determine from the coefficients in this balanced chemical equation is, moles.