The given balanced reaction is,
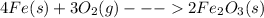
The stoichiometric coefficients of each element or compound represents the number of moles of that element or compound required for the complete reaction to take place.
The mole ratios of different products and reactants will be:
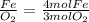
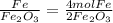
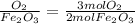
So the mole ratio comparing iron (Fe) and oxygen gas (
 ) is
) is
4 : 3