Answer:
Heat,
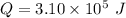
Step-by-step explanation:
Given that,
Mass of water, m = 1 kg
Initial temperature,
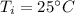
Final temperature,
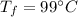
The specific heat of water is,
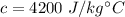
The heat released due to the change in temperature is given by :
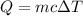
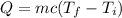
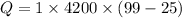
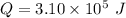
So, the heat input is
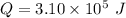 . Hence, this is the required solution.
. Hence, this is the required solution.