Answer: The chemical equation is written below.
Step-by-step explanation:
Aluminium nitrate is formed by the combination of aluminium ions and nitrate ions. This is an ionic compound.
When this compound is dissolved in water, it leads to the ionization of the salt. Ionization of salt is defined as the process in which a salt dissociates into its respective ions.
The chemical equation when aluminium nitrate is dissolved in water follows:
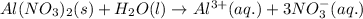
Hence, the chemical equation is written above.