Hello!
To calculate the temperature of 5,85 moles of N₂ gas in a 12,0 L steel bottle under 10,00 atm of pressure we need to use the
Ideal Gas Law. This law relates the pressure and volume of a gas with its temperature and number of moles. The calculations for this exercise are shown below:
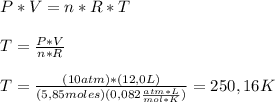
Now we convert this temperature to °C
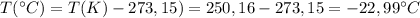
So, the temperature of 5,85 moles of N₂ gas in a 12,0 L steel bottle under 10,00 atm of pressure is
-22,99 °C
Have a nice day!