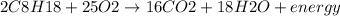Answer:
C. energy
Step-by-step explanation:
The balanced reaction is:
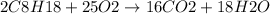
This is essentially a combustion reaction in which a hydrocarbon such as octane in this case, reacts with oxygen to form carbon dioxide and water. Combustion reactions are exothermic which implies that energy is released as the reaction progresses. Thus, the given reaction can be represented by adding the term 'energy' to the product side of the reaction: