Answer:
Carbon-12
Step-by-step explanation:
Let's consider Avogadro's number: 1 mole of atoms has 6.02 × 10²³ atoms.
The molar mass of the isotope carbon-12 is 12 g/mol. The atoms contained in 1 g of ¹²C are:
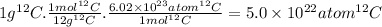
The molar mass of the isotope carbon-13 is around 13 g/mol. The atoms contained in 1 g of ¹³C are:
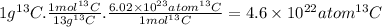
There are more atoms in a 1-g sample of ¹²C than in a 1-g sample of ¹³C.