Hello!
0,00128 moles of Sodium Chloride must be added to the solution to completely precipitate 0,133 g of dissolved Lead.
The chemical reaction for the precipitation of lead from an aqueous solution is:
Pb⁺²(aq) + 2NaCl(aq) → PbCl₂(s) + 2Na⁺(aq)
To calculate how many moles of NaCl are needed, we'll use the following conversion factor to go from grams of Pb⁺² to moles of NaCl using atomic masses and reaction coefficients:
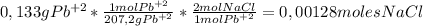
Have a nice day!