Answer:
176 L liters of water vapor can be produced.
Step-by-step explanation:
Using the Ideal gas law,
PV = nRT, where P, T, n, R and T stand for pressure, volume, moles, universal gas constant and temperature respectively.
n = m/M, where n, m and M stand for moles, mass and molar mass respectively.
PV =
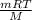
Given ,
T = 312 K
P = 0.98 atm
m = 108 g
M (CH4) = 16.04 g /mol
R = 0.0821 Latm/molK
V =
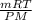
Plugging the numbers in the Ideal gas law we get,
V =
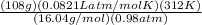
V = 175.99 L