The given salt of weak acid and strong base will hydrolyze as

For this the equilibrium constant will be "Kb"
![Kb=([CN^(-)][OH^(-)])/([CN^(-)])](https://img.qammunity.org/2019/formulas/chemistry/high-school/4ujngl70iwh20a2kf541z8siuiejrg4k3j.png)
The relation between Kb and Ka is
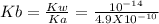
Let the amount of salt hydrolyzed is "x"
Therefore [HCN] = [OH-]=x
[CN-]=0.135-x
Putting values
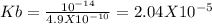
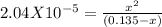
WE may ignore x in denominator as Kb is very low
On solving
x = 1.66X10⁻³ =[OH⁻]
pOH = -log[OH⁻] = -log(1.66X10⁻³)=2.78
Therefore
pH = 14 - pOH = 14-2.78 = 11.22