Hello!
The initial mass of
Magnesium Sulfate Heptahydrate (MgSO₄·7H₂O) is 23,08 g
The chemical reaction for the dehydrating of
Magnesium Sulfate Heptahydrate (MgSO₄·7H₂O) is the following:
MgSO₄·7H₂O(s) + Δ → MgSO₄(s) + 7H₂O(g)
We know that the sample loses 11,80 g upon heating.
That mass is the mass of Water that is released as vapor. Knowing that piece of information, we can apply the following conversion factor to go from the mass of water to the moles of water and back to the mass of the original compound (mi).
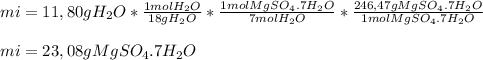
Have a nice day!