Hello!
The ball took up 2,9L
To solve this problem, we should apply the
ideal gas law and clear for n (the number of moles), as this value doesn't change when Devin takes the ball to the garage:
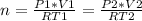
Now, we should clear this equation to find V2, as this is the value we are looking for:
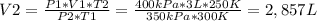
≈2,9L
Have a nice day!