Hello!
To answer this question you need to first write the chemical equation for this reaction:
2Na(s) + Cl₂ → 2NaCl
Now, you know that to react 1 mol of Chlorine gas you'll need 2 moles of sodium, this can be written as follows:
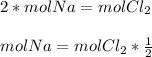 (1)
(1)So, the last step in solving this problem is to know how many moles of Chlorine you have. For that, you'll use the Ideal Gas Law which states that:
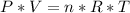
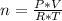 (2)
(2)Where P is pressure, V is volume, n is the amount of the gas in moles, R is the Constant of the Ideal Gases and T is the temperature.
The final step is replacing the expression
(2) in
(1) and you're good to go!
I won't give the answer to this problem, just leave the equation for you to replace the values.