Answer:
Density of the mineral is equal to
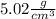
Step-by-step explanation:
The density of a substance is equal to mass of the substance divided by its volume.
Thus, the formula of density (d) is as follows
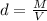
Where, M represent the mass of a substance
and V represents the volume of the same substance
Substituting the given values in above equation, we get -
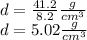
Hence, option B is correct