For the calculation of moles, we divide mass of the substance given by the molar mass of the substance.
Its mathematically representation is as follows:
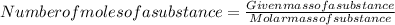
Here given mass of substance is 1.09 kg that is 1090 g
Molar mass of the substance is 78 g/mol
So number of moles can be calculated as:
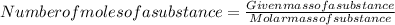
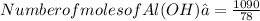
So number of moles of Al(OH)₃ =13.9 mol