Answer:
F gains one electron.
Step-by-step explanation:
Hello,
Due to the high electronegativity of the fluorine atom (approx 3.98), it tends to gain electrons rather than lose them. Now, as lithium has the following electron configuration:
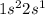
It loses one electron that is gained by the fluorine to form lithium fluoride, LiF.
Best regards.