Answer: Option (a) is the correct answer.
Step-by-step explanation:
Reduction is a process in which there is loss of oxygen atom from a molecule. Also in reduction there is a decrease in oxidation state of the metal.
Whereas in oxidation process there is gain of oxygen atom.
In the given reaction,
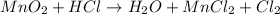
Here, oxidation state of manganese reduces from +4 to +2 and also there in loss of oxygen form manganese oxide molecule to form manganese chloride.
Thus, we can conclude that Mn has been reduced.