Answer: 11,000 J
Step-by-step explanation:
In an isothermal process,
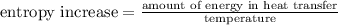
(1)
Note that, the energy used in heat transfer is not available for work. So, the amount of energy unavailable for work is equal to the energy used in heat transfer.
To obtain the amount of energy in heat transfer, we multiply both sides of equation (1) by the denominator of the right side of (1) so that
amount of energy in heat transfer = (entropy increase)(temperature)
= (25 J/K)(440 K)
= 11,000 J
Since the amount of energy unavailable for work is equal to the amount of energy in the heat transfer, therefore the amount of energy unavailable for work is 11,000 J.