Answer:
B. 262 kPa
Step-by-step explanation:
First we must find the total of moles in the container:
3 moles of helium + 2 moles of oxygen + 1 mole s of carbon = 6 moles.
of the total of moles, 2 are oxygen, so the fraction of oxygen in the container is
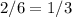
Thus, the partial pressure of the oxygen gas must be a third of the total pressure in the container:
Partial pressure of the oxygen gas =
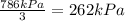
The answer is B. 262 kPa