Answer:
C. Keq will increase
Step-by-step explanation:
Endothermic means the reaction will absorb heat to occur, hence an increase in temperature will favor the reaction, increasing the formation of products.
Keq is the equilibrium constant of the reaction, and it can be defined as:
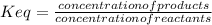
In an endothermic reaction the equilibrium will be shift towards the formation of products when the temperature is increased, the concentration of products will increase (in relation to the concentration of reactants) ; so as a result the equilibrium constant Keq will also increase.