The problem is based on Charles law.
The law states that, at constant pressure the volume occupied by an ideal gas varies directly with its temperature in Kelvin.
The equation for the law can be written as
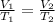
We have been given that
V₁ = 8.98 dm³
V₂ = ?
T₁ = 38.8 °C.
To convert this to Kelvin , we add 273.15.
Therefore T₁ = 311.95 K
T₂ = 233.25 K
Let us plug the values in the Charles equation.
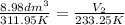
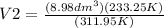
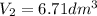
The gas will occupy 6.71 dm³ at -39.9 °C if the pressure remains constant.