1 mole of any substance corresponds to a special number called "Avogadro's Number" (6.022×10²³ (atoms, molecules, ions) ). The units of this number vary depending upon circumstance. In this problem for example, you are asked about a mole of
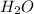
which is a molecule. Therefore, your units must be molecules rather than atoms or ions.
Answer:There are 6.022×10²³
molecules of
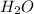
in 1 mole of
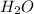
.