Step-by-step explanation:
Magnesium carbonate reacts with nitric acid to give magnesium nitrate and carbonic acid.
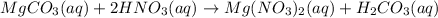
This type of reaction is termed as double displacement reaction, in which ions of compounds changes their positions with each other to produce the new products.
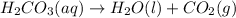
This type of reaction is termed as decomposition reaction, in which a compound breaks down into two or more smaller compounds.