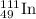Answer: The nuclide is
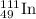
Explanation: We are given a radioactive nuclide having 46 protons and 62 neutrons.
In an element,
Atomic number = number of protons = number of electrons
Hence, Atomic number = 49
and, Atomic mass = number of neutrons + number of protons
Atomic mass = 62 + 49 = 111
An element having atomic number 49 is Indium (In).
Representing an element =
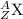 where, X = element
where, X = element
A = Atomic mass
Z = Atomic number
Radioactive isotope used to label blood platelets is