Answer: Option (c) is the correct answer.
Step-by-step explanation:
A compound which is formed by chemical combination of two elements is known as a binary compound.
For example,
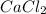 is a binary compound as it contains two elements calcium and chlorine.
is a binary compound as it contains two elements calcium and chlorine.
Therefore, decomposition reaction of this binary compound will be as follows.

As a decomposition reaction is defined as a reaction which yields to the formation of two or more number of products.
Here, the constituent elements of the reactant are calcium and chlorine.
Thus, we can conclude that the statement reactants are the constituent elements, is true about the decomposition of a simple binary compound.