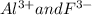In aluminium fluoride, aluminium carries positive charges so fluoride should carry negative charge to be electrically neutral.So for aluminium ion with three positive charges fluoride ion should possess three negative charges to make aluminium fluoride electrically neutral.
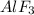 →
→