Answer: The correct answer is Option A.
Step-by-step explanation:
We are given some ions, which are:
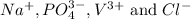
The compounds which may be formed from these ions will be
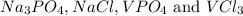
Precipitates are defined as the substances which are not soluble in water at any temperatures. They remain undissolved in water.
Out of the formed salts, the soluble ones are
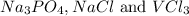
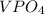 remains insoluble in water and thus, the correct answer is Option A.
remains insoluble in water and thus, the correct answer is Option A.