Answer:

Step-by-step explanation: According to Avogadros law, one mole of any substance contains a fixed number of particles i.e
 particles (atoms, molecules or ions) which is also known by the name Avogadro's number.
particles (atoms, molecules or ions) which is also known by the name Avogadro's number.
For example: 1 mole of
 molecule contains
molecule contains
 molecules of oxygen.
molecules of oxygen.
1 mole of
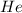 contains
contains
 atoms of helium.
atoms of helium.