Step-by-step explanation:
Law of conservation of mass states that mass can neither be destroyed nor it can be created in a chemical reaction. It can only be transformed from one form to another.
Therefore, when we write a chemical reaction then it is necessary to balance the chemical equation because the total number of reactants participating in a reaction will yield the same number of products.
Hence, a chemical equation can only be balanced when total number of reactants equal total number of products. Thus, it resembles the law of conservation of mass.
For example,
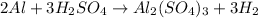 is a balanced chemical equation because the number of aluminium, hydrogen, and sulfate atoms on both reactant and product side are the same.
is a balanced chemical equation because the number of aluminium, hydrogen, and sulfate atoms on both reactant and product side are the same.