Answer: False
Explanation: In a single replacement reaction, one metal can only be replaced by another metal if the metal which is replaced is less reactive than the metal coming to take its place. For example, Cu is less reactive than Zn. So, Cu could easily be replaced by Zn.
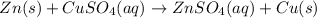
Pb is less reactive than Al and so it can not replace Al from
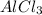 .
.
Hence, the given statement is false.