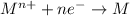Answer: electrons
Step-by-step explanation:
Oxidation-reduction reaction or redox reaction is defined as the reaction in which oxidation and reduction reactions occur simultaneously.
Oxidation reaction is defined as the reaction in which a substance looses its electrons. The oxidation state of the substance increases.
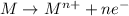
Reduction reaction is defined as the reaction in which a substance gains electrons. The oxidation state of the substance gets reduced.