Answer: The volume of tetrahydrofuran the student should pour out is 33.7 ml
Step-by-step explanation:
Density is defined as the mass contained per unit volume.
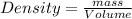
Given : Mass of tetrahydrofuran = 30.0 grams
Density of tetrahydrofuran =
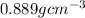
Putting in the values we get:
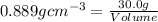
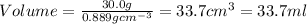
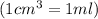
Thus volume of tetrahydrofuran the student should pour out is 33.7 ml