Answer:- Mass of the titanium alloy is 7.01 g, choice C is correct.
Solution:- The heat of fusion is given as 422.5 joules per gram and it also says that 2960 joules of heat is required to melt the metal completely.
The suggested equation is,
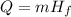
where Q is the heat energy, m is the mass and Hf is the heat of fusion.
Since, we are asked to calculate the mass, the equation could be written as:
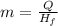
Let's plug in the values in it:
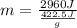
m = 7.01 g
So, the mass of the titanium alloy is 7.01 g, choice C is correct.