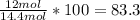Given the balanced chemical equation for combustion of propane:
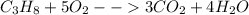
Moles of propane
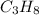 =3.6 mol
=3.6 mol
Propane is the limiting reactant as the other reactant oxygen is said to be present in excess.
Amount of products formed would depend on the moles of limiting reactant.
Mole ratio of water to propane as per the balanced chemical equation
=
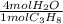
Calculating the moles of water produced from 3.6 mol propane:
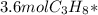
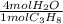
= 14.4mol

Theoretical yield of water = 14.4 mol
Actual yield = 12 mol
Percent yield =
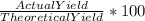
=