Answer:
The correct option is: 13305 years
Explanation:
Formula for radioactive decay is:
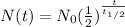 , where
, where
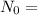 Initial amount,
Initial amount,
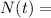 Final amount after
Final amount after
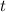 years and
years and
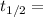 Half-life in years.
Half-life in years.
Given that, the sample from a fossil has 2 grams of carbon 14 and it had 10 grams of carbon 14 at the moment of its demise.
That means,
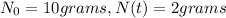
The half-life of carbon 14 is 5730 years. So,
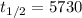 years.
years.
Now according to the above formula, we will get......
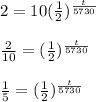
Taking logarithm on both sides......
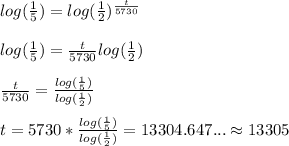
Thus, the age of the fossil is 13305 years.