Answer:
Phosphorus acid
Step-by-step explanation:
Hi, as can be seen it's an acid of phosphorus.
Looking the charge of the ion you can determine the valence number of the phosphorus: If there are 3
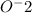 (equal -6) and the total charge is -3 :
(equal -6) and the total charge is -3 :
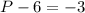
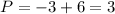
So it's a phosphorus acid (valence=3) and you can see it's structure in the Figure