Answer :
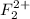 is paramagnetic.
is paramagnetic.
Explanation :
According to the molecular orbital theory, the general molecular orbital configuration will be,
![(\sigma_(1s)),(\sigma_(1s)^*),(\sigma_(2s)),(\sigma_(2s)^*),[(\pi_(2p_x))=(\pi_(2p_y))],(\sigma_(2p_z)),[(\pi_(2p_x)^*)=(\pi_(2p_y)^*)],(\sigma_(2p_z)^*)](https://img.qammunity.org/2019/formulas/chemistry/college/rf5czo06upvtqw57oe5qafkcp3g0sf37gt.png)
As there are 9 electrons present in fluorine.
The number of electrons present in
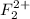 molecule = 2(9) - 2 = 16
molecule = 2(9) - 2 = 16
The molecular orbital configuration of
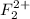 molecule will be,
molecule will be,
![(\sigma_(1s))^2,(\sigma_(1s)^*)^2,(\sigma_(2s))^2,(\sigma_(2s)^*)^2,(\sigma_(2p_z))^2,[(\pi_(2p_x))^2=(\pi_(2p_y))^2],[(\pi_(2p_x)^*)^1=(\pi_(2p_y)^*)^1],(\sigma_(2p_z)^*)^0](https://img.qammunity.org/2019/formulas/chemistry/college/agdrkku4iwgy0u0v9tl80w0lrimvjutngl.png)
Paramagnetic compounds : They have unpaired electrons.
Diamagnetic compounds : They have no unpaired electrons that means all are paired.
The number of unpaired electron in molecule is, 2. So, this is paramagnetic. That means, more the number of unpaired electrons, more paramagnetic.
Thus, is paramagnetic.