Answer : The molecule
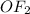 is a polar molecule.
is a polar molecule.
Explanation :
Polar molecule : When the arrangement of the molecule is asymmetrical then the molecule is polar.
Non-polar molecule : When the arrangement of the molecule is symmetrical then the molecule is non-polar.
The given molecule is,
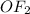
The electronegativities of oxygen and fluorine are different. The molecular geometry of
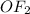 is bent. As, Fluorine is more elctronegative than the oxygen. So, the arrows putting towards the more electronegative element i.e, fluorine. These arrows do not balance each other. Due to this, the asymmetrical arrangement of these bonds makes the molecule polar.
is bent. As, Fluorine is more elctronegative than the oxygen. So, the arrows putting towards the more electronegative element i.e, fluorine. These arrows do not balance each other. Due to this, the asymmetrical arrangement of these bonds makes the molecule polar.
Hence, the given molecule
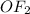 is polar.
is polar.