Answer: We will use the electron-dot structure to tell the reactivity of fluorine.
Explanation: Fluorine atom is the 9th element of the periodic table.
Atomic number: 9
Electronic configuration of fluorine =
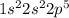
The number of valence electrons = 7
and the valency for this element = 1 (because this element requires only one electron to have a stable electronic configuration)
Electron dot structures are the structures which represent the valence electrons present around the nucleus of an atom. The dots in the structures represent the electrons.
Reactivity of an element is defined as its ability to gain or loose electrons easily.
As fluorine atom gains 1 electron easily, so it is very reactive.
Electron dot structure of fluorine atom is given in the image attached.