Answer:
σ*2pₓ, also called
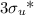
Explanation:
I have drawn the MO diagram for fluorine below.
Each F atom contributes seven valence electrons, so we fill the MOs of fluorine with 14 electrons.
We have filled the
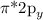 and
and
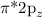 MOs.
MOs.
They are the highest occupied molecular orbitals (HOMOs).
The next unfilled level (the LUMO) is the σ*2pₓ orbital. If you use the symmetry notation, it is called the
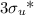 orbital.
orbital.
This is the orbital that fluorine uses when it acts as an electron acceptor.