Answer:-
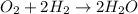 and
and
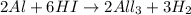
Explanations:- In the first equation, the oxidation number of O is decreasing from 0 to -2 where as the oxidation number of H is increasing from 0 to +1. As the oxidation number is increasing for one element(H) and decreasing for other element(O), it is an oxidation-reduction reaction.
Second reaction is a double displacement reaction so there is no change to the oxidation numbers of the elements and hence it is not oxidation-reduction reaction.
In third reaction, the oxidation number of Al is increasing from 0 to +3 and the oxidation number of H is decreasing from +1 to 0. Here again there is an increase and decrease to the oxidation numbers of two elements, it is oxidation-reduction reaction.
fourth and fifth reactions are again double displacement reactions and so there is no change to oxidation numbers for any of the elements and they are not the oxidation-reduction reactions.
So, the only correct equations are
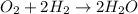 and
and
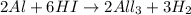 .
.