Answer: C. The chunk of iron's volume is smaller than the chunk of aluminum's volume.
Explanation:
Given: The mass of aluminum chunk = 10 kg
In grams , the mass of aluminum chunk = 1000 g [∵ 1 kg = 1000 g]
The mass of iron chunk =10 g
i.e The chunk of aluminum is more massive than the chunk of iron.
Density of Aluminum chunk =
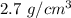
Density of iron chunk =
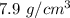
The formula of volume is given by :-
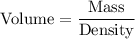

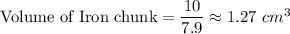
Clearly, The chunk of iron's volume is smaller than the chunk of aluminum's volume.