Answer : The correct answer is 3.
Explanation :
The given chemical reaction is,
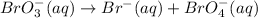
In a disproportionate reaction, disproportionate substance is a single substance which is oxidized as well as reduced.
The oxidation state of Br in
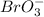 is (+5).
is (+5).
The oxidation state of Br in
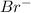 is (-1).
is (-1).
The oxidation state of Br in
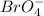 is (+7).
is (+7).
In the given chemical reaction,
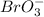 is getting reduced to
is getting reduced to
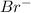 and
and
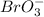 is getting oxidized to
is getting oxidized to
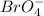 . So, bromine is a disproportionate substance.
. So, bromine is a disproportionate substance.
That means the bromine is present in three oxidation states.
Therefore, three oxidation states of the disproportionate substance have throughout the reaction.