Fluorine has C seven valence electrons.
Valence electrons are the electrons that are found on the outer shell of an atom that are capable of participating in chemical reactions. The easiest way to figure out how many valance electrons Fluorine has would be to look it up in a periodic table and notice that it a group 7 element and therefore has 7 valence electrons.
The other way to tell that Fluorine has 7 valence electrons is to notice that an element with an atomic number of 9 has 9 electrons. The electronic configuration of elements has the first 2 electrons go on the first shell or energy level
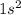 , the leaving the next 7 to go to the second shell
, the leaving the next 7 to go to the second shell
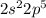 which can take up to 8 electrons.
which can take up to 8 electrons.
This means that Fluorine has 7 seven valence electrons.